Designed to meet the specific demands of the biotech and pharmaceutical industries, LKH UltraPure centrifugal pumps deliver maximum uptime and high efficiency plus the benefits of easy cleanability and thorough documentation.
The LKH UltraPure is the premier choice for biopharmaceutical processes that demand high efficiency, exceptional cleanability, contamination safety, robust design and low maintenance. An external shaft seal, advanced impeller design and crevice-free internal surfaces are the special features, which make these pumps ideal for ultra-clean processes where low energy consumption is of concern.
LKH is also available in a wide rang of other configurations for food, beverage, high-pressure applications etc.
The LKH UltraPure has three design finesses that contribute to an ultraclean process environment.
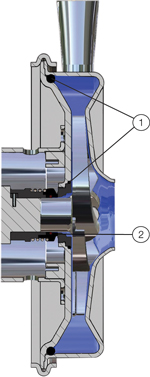
Specially designed semi-open impeller with balance holes that optimize the flow on the back of the impeller and contribute to the high cleanability of the shaft seal as well as of the pump itself. Increased flow around the shaft seal also provides good cooling and circulation, which prolong the service life of the seal.
The number of product wetted parts is limited to a minimum. The few parts are perfectly matched and joints are sealed with o-rings that are tightened with a pre-defined compression, leaving no room for contaminants to hide and minimizing the elastomer surfaces in contact with the product.
External shaft seal provides maximum hygiene by placing difficult-to-clean parts on the outside of the pump. The external shaft seal ensures that the centrifugal forces in the pump contribute to the enhanced lubrication of the shaft seal and that wear particles are forced out of the pump and do not mix with the product.
High efficiency, optimized flow, low contamination risk and minimal wear are hallmarks of the LKH UltraPure.
A precision-engineered pump and motor provide optimal efficiency with gentle product treatment. The robust design and tight tolerances together with the advanced impeller design minimize slip and ensure that the energy is used to transport the liquid through the pump without diminishing cleanability.
The advanced impeller design provides optimal fluid flow throughout the pump. High flow, especially behind the impeller where the shaft seal is located, contributes to easy cleanability and enhanced lubrication and cooling of the shaft seal. The specially designed inlet with the vanes on the impeller blades contributes energy to the flow of fluids prior to entering the pump casing. This minimizes the NPSHr (Net Positive Suction Head required) as well as the forces on the product itself.
The external shaft seal ensures that wear particles from the shaft seal are directed by centrifugal force to the outside and thus do not come into contact with the medicinal product. The low NPSHr minimizes the risk of cavitation, which can damage shaft seals and pump components and introduce contaminants into process fluids. Carefully selected materials and easy-to-clean design also reduce the risk of contamination.
The semi-open impeller design together with the external shaft seal maximizes flow and helps cool and lubricate the shaft seal. This prolongs the service life of the seal.
The LKH UltraPure comes in five different standard sizes with capacities of up to 90 m3/hour. A smart alternative for pump duties that require continuous operation, the LKH UltraPure centrifugal pump delivers the highest possible efficiency and minimizes energy consumption. It is also available with a wide range of options for:
To shorten lead times, all standard pumps are in stock to ensure fast and efficient delivery within 10 working days. Delivery of standard pumps with any option is 20 working days from order receipt.
All equipment and components in the Alfa Laval BioPharm portfolio are supplied with Alfa Laval Q-doc, a comprehensive documentation package that provides full transparency of the entire supply chain, from raw material to final equipment delivery. This smoothes purchasing and installation procedures as well as facilitates qualification, validation and change control procedures. Based on GDP (Good Documentation Practice), Alfa Laval Q-doc covers every aspect of BioPharm equipment supply and provides customers with transparent and well-documented quality assurance of the sourced equipment.
The Q-doc package for LKH UltraPure comprises equipment manuals, performance tests, quality and manufacturing procedures, material certificates, traceability of product wetted parts and the necessary parts and service information. This attention to detail maximizes uptime and minimizes risk.