Penerangan
【SUMMARY】
3,4-methylenedioxypyrovalerone (MDPV) is a psychoactive recreational drug with stimulant properties which acts as a norepinephrine-dopamine reuptake inhibitor (NDRI). It was first developed in the 1960s by a team at Boehringer Ingelheim1. MDPV remained an obscure stimulant until around 2004 when it was reportedly sold as a designer drug. Products labeled as bath salts containing MDPV were previously sold as recreational drugs in gas stations and convenience stores in the United States, similar to the marketing for Spice and K2 as incense.
MDPV is the 3,4-methylenedioxy ring-substituted analog of the compound pyrovalerone, developed in the 1960s, which has been used for the treatment of chronic fatigue and as an anorectic, but caused problems of abuse and dependence. However, despite its structural similarity, the effects of MDPV bear little resemblance to other methylenedioxy phenylalkylamine derivatives such as 3,4-methylenedioxy-N-methylamphetamine (MDMA), instead producing primarily stimulant effects with only mild entactogenic qualities.
MDPV undergoes CYP450 2D6, 2C19, 1A2, and COMT phase 1 metabolism (liver) into methylcatechol and pyrrolidine, which in turn are glucuronated (uridine 5'-diphospho-glucuronosyl-transferase) allowing it to be excreted by the kidneys, with only a small fraction of the metabolites being excreted into the stools3. No free pyrrolidine will be detected in the powder.
The 3, 4-methylenedioxypyrovalerone (MDPV) assay contained within the Rapid Test Panel yields a positive result when the methamphetamine concentration in powder exceeds 1000ng/mL.
【DIRECTIONS FOR USE】
Test panel (in closed pouches), samples, and controls should be brought to room temperature (15-30°C) prior to testing. Do not open pouches until ready to perform the assay.
Remove the test panel from its protective pouch and label the panel with patient’s identification or control label.
FOR SURFACES
1. Wipe with the sample marked side of strip in the panel over the surface on which the drugs are suspected.
2. Take off the cap of buffer vial.
3. Fill all buffer from the buffer vial into the protection cover.
4. Insert the Test slowly and carefully into the protection cover with buffer.
5. Wait for control line to appear on the membrane, read the result after 5 minutes and do not interpret the result after 10 minutes.
_Rapid_Test_Panel___Powder(1).jpg)
FOR SOLIDS WITHOUT SWAB
1. Open the buffer vial and put a pinch of suspected solid into the buffer.
2. Close the vial. Shake it vigorously for a short time. Wait for 30sec.
3. Take off the dropper cap.
4. Fill all the buffer with dissolved substances into the protection cover.
5. Insert the Test slowly and carefully into the protection cover with buffer.
6. Wait for control line to appear on the membrane.
7. Read the results after 5 minutes and do not interpret the result after 10 minutes.
_Rapid_Test_Panel___Powder1(1).jpg)
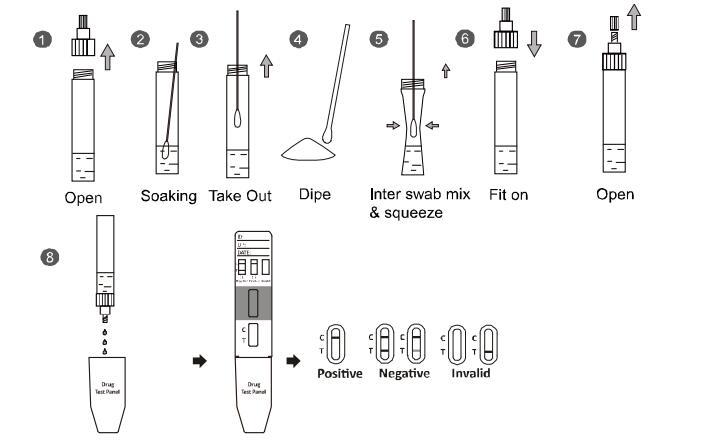
FOR SOLIDS WITH SWAB
1. Open buffer vial. Insert the swab into the tube for 5-10 seconds to allow swab head to be thoroughly soaked in the buffer.
2. Take out the swab and wipe the surface solids gently at least 3 times, and then insert the swab into buffer vial and mix well for at least 1 minute. Squeeze the swab several times by compressing the outside walls of the vial against the swab to mix well. Finally squeeze the swab to make most of the solution stay in the buffer vial and remove the swab. Use solution thus obtained as specimen.
3. Take off the cap of dropper cap.
4. Fill the solution with dissolved substances into the protection cover.
5. Insert the Test Panel slowly and carefully into the protection cover with buffer.
6. Wait for control line to appear on the membrane.
7. Read the result after 5 minutes and do not interpret the result after 10 minutes.
|
Cat. No.
|
Product Description
|
Specimen
|
Format
|
Kit Size
|
Cut-Off
|
Status
|
|
DMDP-X14
|
Methylenedioxypyrovalerone (MDPV) Rapid Test Panel
|
Powder
|
Panel
|
25 T
|
1000 ng/mL
|
CE
|
Lebih maklumat tentang Setia Scientific Solution
_Rapid_Test_Panel___Powder(1).jpg)
_Rapid_Test_Panel___Powder1(1).jpg)

 Malaysia
Malaysia