Description
【SUMMARY】
The HIV 1.2.O Rapid Test (Whole Blood/Serum/Plasma): HIV (Human Immunodeficiency Virus) is the etiologic agent of Acquired Immune Deficiency Syndrome (AIDS). The virion is surrounded by a lipid envelope that is derived from the host cell membrane. Several viral glycoproteins are on the envelope. Each virus contains two copies of positive-sense genomic RNAs. HIV-1 has been isolated from patients with AIDS and AIDS-related complex, and from healthy people with high potential risk for developing AIDS. HIV-1 consists of Subtype M and Subtype O. Highly divergent strains of HIV-1 were first recognized in 1990 and grouped provisionally as Subtype O as this variation has similar glycoprotein markers to HIV-1 but a slight variation to the protein marker. Although rarely compared to HIV-1 and HIV-2, infections caused by Subtype O have so far been identified in Africa (Cameroon), France and Germany. HIV-2 has been isolated from West African AIDS patients and from seropositive asymptomatic individuals. HIV-1, HIV-2, and Subtype O all elicit immune responses. Detection of HIV antibodies in serum, plasma or whole blood is the most efficient and common way to determine whether an individual has been exposed to HIV and to screen blood and blood products for HIV. 4 Despite the differences in their biological characters, serological activities and genome sequences, HIV-1, HIV-2, and Subtype O show strong antigenic cross-reactivity. Most HIV-2 positive sera can be identified by using HIV-1 based serological tests.
The Syphilis Rapid Test (Whole Blood/Serum/Plasma) utilizes a double antigen combination of a Syphilis antigen coated particle and Syphilis antigen immobilized on membrane to detect TP antibodies (IgG and IgM) qualitatively and selectively in whole blood, serum or plasma. Treponema Pallidum (TP) is the causative agent of the venereal disease Syphilis. TP is a spirochete bacterium with an outer envelope and a cytoplasmic membrane. Relatively little is known about the organism in comparison with other bacterial pathogens. According to the Center for Disease Control (CDC), the numbers of cases of Syphilis infection has markedly increased since 1985. Some key factors that have contributed to this rise include the crack cocaine epidemic and the high incidence of prostitution among drug users. One study reported a substantial epidemiological correlation between the acquisition and transmission of the HIV virus and Syphilis. Multiple clinical stages and long periods of latent, asymptomatic infection are characteristic of Syphilis. Primary Syphilis is defined by the presence of a chancre at the site of inoculation. The antibodies response to the TP bacterium can be detected within 4 to 7 days after the chancre appears. The infection remains detectable until the patient receives adequate treatment.
【DIRECTIONS FOR USE】
Allow test cassette, specimen, Buffer and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test cassette from the sealed pouch and use it as soon as possible. Best results will be obtained if the assay is performed within one hour.
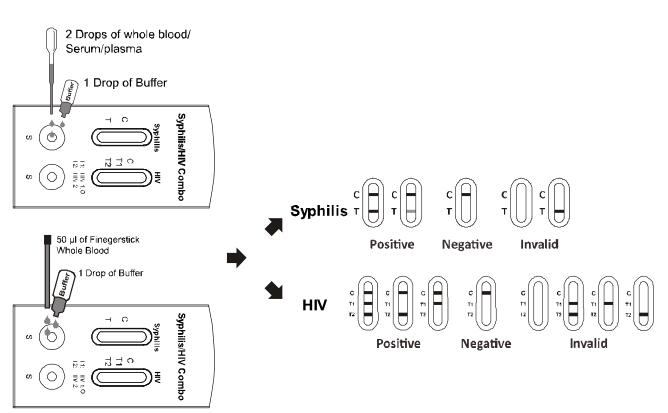
2. Place the test cassette on a clean and level surface.
3. For Venipuncture Whole Blood, serum or plasma specimen:
Place the test cassette on a clean and level surface. Hold the dropper vertically and transfer 2 drops of whole blood, serum or plasma (approximately 50 ul) to the specimen wells, then add 1 drop of buffer (approximately 40 ul), respectively. Start the timer. See the illustration below.
4. For Fingerstick Whole Blood specimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50m mL of fingerstick whole blood specimen to the specimen wells of test cassette, then add 1 drop of buffer(approximately 40 ul) respectively, and start the timer. See the illustration below.
5. Wait for the colored line(s) to appear. The test result should be read at 10 minutes. Do not interpret the result after 20 minutes.
Note:It is suggested not to use the buffer, beyond 30 days after opening the vial.
|
Cat. No. |
Product Description |
Specimen |
Format |
Kit Size |
Cut-Off |
Status |
|
IISC-425 |
Syphilis/HIV1.2.O Combo Rapid Test Cassette |
WB/S/P |
Cassette |
25 T |
See Insert |
Non-CE |
More detail about Setia Scientific Solution

 Malaysia
Malaysia