Description
【SUMMARY】
The HBsAg Rapid Test (Serum/Plasma) is a rapid test to qualitatively detect the presence of HBsAg in serum or plasma specimen. The test utilizes a combination of monoclonal and polyclonal antibodies to selectively detect elevated levels of HBsAg in serum or plasma. Viral hepatitis is a systemic disease primarily involving the liver. Most cases of acute viral hepatitis are caused by Hepatitis A virus, Hepatitis B virus (HBV) or Hepatitis C virus. The complex antigen found on the surface of HBV is called HBsAg. Previous designations included the Australia or Au antigen. 1 The presence of HBsAg in serum or plasma is an indication of an active Hepatitis B infection, either acute or chronic. In a typical Hepatitis B infection, HBsAg will be detected 2 to 4 weeks before the ALT level becomes abnormal and 3 to 5 weeks before symptoms or jaundice develop. HBsAg has four principal subtypes: adw, ayw, adr and ayr. Because of antigenic heterogeneity of the determinant, there are 10 major serotypes of Hepatitis B virus.
The HCV Rapid Test (Serum/Plasma) is a rapid test to qualitatively detect the presence of antibody to HCV in a serum or plasma specimen. The test utilizes colloid gold conjugate and recombinant HCV proteins to selectively detect antibody to HCV in serum or plasma. The recombinant HCV proteins used in the test kit are encoded by the genes for both structural (nucleocapsid) and non-structural proteins. Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA Virus. HCV is now known to be the major cause of parenterally transmitted non-A, non- B hepatitis. Antibody to HCV is found in over 80% of patients with well-documented non-A, non-B hepatitis. Conventional methods fail to isolate the virus in cell culture or visualize it by electron microscope. Cloning the viral genome has made it possible to develop serologic assays that use recombinant antigens. Compared to the first generation HCV EIAs using single recombinant antigen, multiple antigens using recombinant protein and/or synthetic peptides have been added in new serologic tests to avoid nonspecific cross- reactivity and to increase the sensitivity of the HCV antibody tests.
The HIV 1.2 Rapid Test (Serum/Plasma) is a rapid test to qualitatively detect the presence of antibody to HIV 1 and/or HIV 2 in whole blood, serum or plasma specimen. The test utilizes latex conjugate and multiple recombinant HIV proteins to selectively detect antibodies to the HIV 1.2 in serum or plasma. HIV is the etiologic agent of Acquired Immune Deficiency Syndrome (AIDS). The virion is surrounded by a lipid envelope that is derived from host cell membrane. Several viral lycoproteins are on the envelope. Each virus contains two copies of positive-sense genomic RNAs. HIV 1 has been isolated from patients with AIDS and AIDS-related complex, and from healthy people with high potential risk for developing AIDS. 6 HIV 2 has been isolated from West African AIDS patients and from seropositive asymptomatic individuals. 7 Both HIV 1 and HIV 2 elicit immune response. 8 Detection of HIV antibodies in serum, plasma is the most efficient and common way to determine whether an individual has been exposed to HIV and to screen blood and blood products for HIV. 9 Despite the differences in their biological characteristics, serological
activities and genome sequences, HIV 1 and HIV 2 show strong antigenic cross- reactivity. 10,11 Most HIV 2 positive sera can be identified by using HIV 1 based serological tests.
【DIRECTIONS FOR USE 】
Allow the test, specimen, buffer and/or controls to room temperature (15-30°C) prior to testing.
1. Remove the test cassette from the foil pouch and use it within one hour. Best results will be obtained if the test is performed immediately after opening the foil pouch.
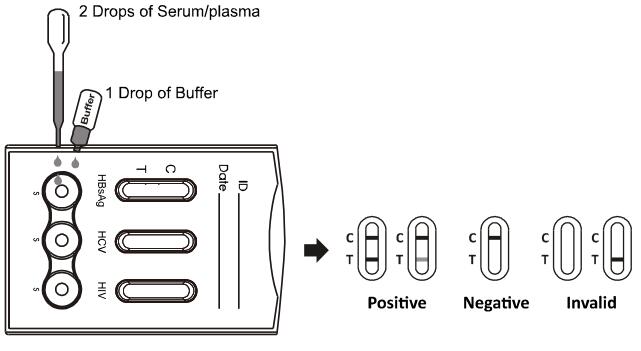
2. Place the test cassette on a clean and level surface. Hold the dropper vertically and transfer 2 drops of serum or plasma (approximately 50 ul) to the each sample well, then add 1drop of buffer (approximately 40 ul) to each sample well and start the timer. See the illustration below.
3. Wait for the colored line(s) to appear. The test result should be read at 10 minutes.
Do not interpret the result after 20 minutes.
|
Cat. No. |
Product Description |
Specimen |
Format |
Kit Size |
Cut-Off |
Status |
|
IBCH-335 |
HBsAg/HCV/HIV Combo Rapid Test Cassette |
S/ P |
Cassette |
25 T |
See Insert |
Non-CE |
More detail about Setia Scientific Solution

 Malaysia
Malaysia